
Online Monitoring reports
Online QA scheduling
File archive
File repository for TMF
An integrated solution
to manage your clinical trial
Features
- Online Monitoring reports
- Online QA scheduling
- File archive
- File repository for Trial Master File
- SOP Management
- 21 CFR Part 11 Compliance
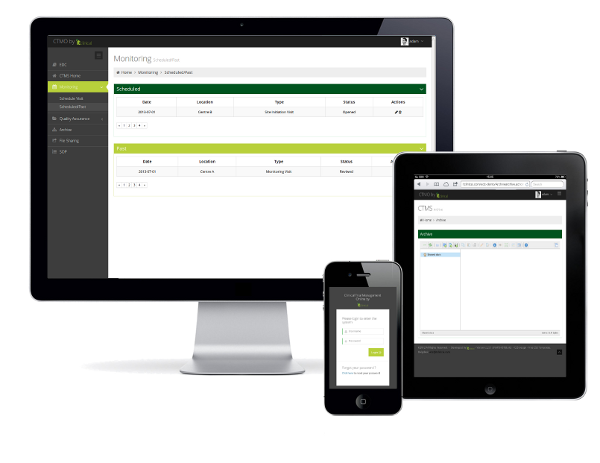
- Responsive layout, works in Smartphones, Tablets and PCs
The Clinical Trial Management System (CTMS) centralizes relevant clinical trial information and allows it to be shared among all trial stakeholders.
The CTMS can be a one-stop-shop for clinical trial related activities for the sponsor, investigators and monitors.
The CTMS can be deployed stand-alone or be coupled with our EDC system.
- Access the clinical trial information from anywhere
- Reduce overall clinical trial data management time
Highlights
Online Monitoring Reports
Online Monitoring Reports
Online QA Reports
Monitors can create reports for Site qualification, Site Initiation, monitoring and closeout visits online. These reports can be customized if you have specific needs.
QA Reports
Quality Assurance can also centralize their work by filling their reports directly in the CTMS.
File Archive
Share files with stakeholders
Electronic Trial Master file
A shared file archive location is ideal to share clinical trial related documents with stakeholders. As a centrally located service, all users will be able to share and access important trial documents.
Electronic Trial Master File
A separate file section is ideal to create an electronic Trial Master File archive for your trial. Access to these documents can be restricted to the users you define.
SOP Management
Manage active/obsolete SOPs
Attach SOP copies
The SOP section is designed to add, list and retrieve SOPs. You can use it to centralize access to your trial's SOPs
With the ability to add files, this feature can help you centralize and share access to the relevant SOPs amongst all stakeholders.
Compliance
21 CFR Part 11 Compliant
Exportable Audit trail
Several user access levels
As in our other tools, the CTMS complies with regulatory requirements for electronic systems.
A thorough audit trail system registers every change
in the system.
A flexible authentication and authorization system allows restricting access to the system and to the functionalities each user can
perform.
By collecting data as electronic source data, transcription errors decrease and trial data accuracy is optimized.
Tailoring to your needs
We provide various deployment options, depending on your infrastructure:
Technology Transfer run system on your network
Hosted solution use system in the cloud
Do you have any specific needs in your trial? We can adapt our base product to meet specific requirements, tailoring the application to you.
Instead of offering a fully fledged solution, we try to understand what you need and provide you with a customized, cost-effective solution.
