
Features
- Adverse Event repository
- CIOMS generation
- E2B XML import/export
- Product registration and management
- PSUR Scheduling, management and generation
- MedDRA integration
- 21 CFR Part 11 Compliance
The pharmacovigilance software is an integrated system that allows the centralization of the major pharmacovigilance aspects, such as Adverse Reactions from post-marketing products and clinical trials, Periodic Safety Update Reports, Licensing Agreements and Medicinal Products database.
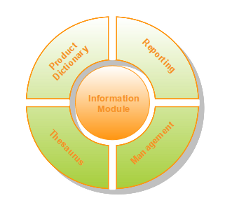
The pharmacovigilance system is comprised of 5 modules. The modularization allows a high level of customization, giving you complete freedom of choice over which modules should be implemented based on your organization's needs.
- Information Module
- Product Dictionary Module
- Reporting Module
- Thesaurus Module
- Management Module
Highlights
centralize your information
Manage/access AE/ADRs
Export to CIOMS/XML
Manage follow-ups
Duplicate detection algorithm
Case narrative generation
Based on ICH's E2B
The information module is the central unit of the pharmacovigilance system. It allows users to enter and manage suspected adverse event cases (AE/ADRs), browse and search existing cases.
The Adverse Event information recorded complies with ICH's E2B, thus providing seamless communication with international regulators' systems, such as EudraVigilance.
PSUR Reporting
PSUR calendar (monthly and yearly views)
Calendar export
Manage delivery dates to multiple countries
Schedule PSURs
Generate PSURs
Compliant with EMA's GVP
The module for your organisation to centralize PSUR management activities, from registration and management of upcoming PSUR submissions to generation of PSUR templates.
PSUR Calendar
The PSUR Calendar is a view of upcoming PSURS. It allows users to understand which PSURS are in need of preparation and delivery on a month-to-month or year-to-year basis.
PSUR Manager
PSUR Manager, the centre of PSUR management, includes historic information about a product's PSURs and scheduling of new PSURs.
PSURs are automatically loaded with the relevant AE/ADRs and the PSUR section templates are created and exported automatically to streamline the PSURs writing activities!
authorized and development drugs
Register active substances and pharmaceutical products
Store authorized and Development products
Manage authorization Dossiers of products in multiple countries
Manage Licensing Partners
Based on ICH's E2C
The Product Dictionary Module supports the Information and Reporting modules.
Gathering the necessary information about the products for purposes of notification of an Adverse Reactions, the organisation can centralize the information of its Development and authorized Products.
Thesaurus Module
Import standard medical terms thesaurus
Manage thesaurus versions
Part of the standard for reporting adverse events is the MedDRA® thesaurus for classification of adverse events.
The thesaurus module aims at integrating this system of classification for seamless compatibility with EudraVigilance.
Dictionaries are also categorized by version, so that new versions of MedDRA® do not compromise the quality of the data already in the system.
Management Module
21 CFR Part 11 Compliant
Exportable Audit trail
Several user access levels
By complying with regulatory requirements for electronic systems the Pharmacovigilance replaces paper based records.
The management module allows users with sufficient privileges to perform administration tasks.
A thorough audit trail system registers every change in the system.
A flexible authentication and authorization system allows restricting access to the system and to the functionalities each user can perform.
By collecting data as electronic source data, transcription errors decrease and trial data accuracy is optimized.
Tailoring to your needs
We provide various deployment options, depending on your infrastructure:
Technology Transfer run system on your network
Hosted solution use system in the cloud
ITClinical can adapt its base product to meet specific requirements, tailoring the application to your needs.
Instead of offering a fully fledged solution, we try to understand what you need and provide you with a customized, cost-effective solution.
Running on Linux servers, this also means there will be no increased licensing costs.
