

Features
- eCRF for remote data entry
- Configurable Edit Checks
- CRF reviewing for monitors
- Online Querying
- Following the inclusion of patients by site in real time by the monitor/sponsor
- Ability to use Double data entry
- 21 CFR Part 11 Compliance
- Responsive layout, works in Smartphones, Tablets and PCs
ITClinical's Electronic Data Capture (EDC) solution centralizes access to clinical trial's patient data. The ability to handle multiple clinical trials simultaneously makes it the central stop for clinical trial patient collection and retrieval.
A Phase I module boosts the base EDC solution with features that will increase work performance for Phase I CROs.
This tool is part of ITClinical's pharmaceutical portfolio and it incorporates the company's philosophy: making life easier for our partners.
By complying with regulatory requirements for electronic systems the EDC replaces paper based records.
- Collaborate online for faster feedback
- Access your data from anywhere
- Reduce overall clinical trial data management time
Highlights
eCRF
Manage eCRF data
Powerful edit checks
Issue and manage queries online
The eCRF module is the central unit of the EDC system. It allows users to collect and manage subject data and export it (including in PDF format for trial master file and trial report).
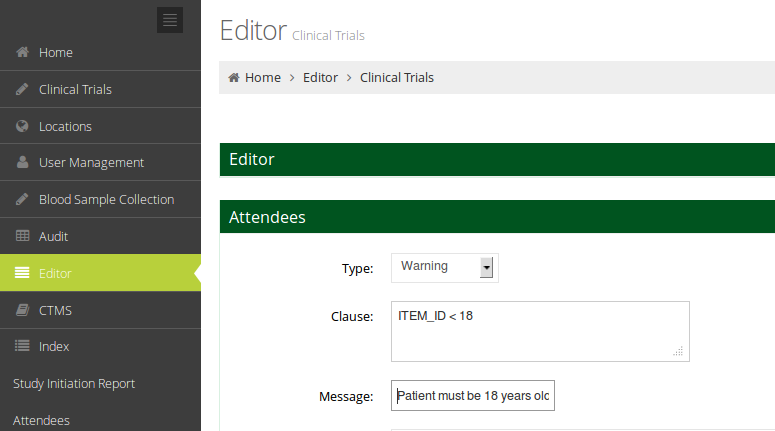
Powerful Edit Checks
Edit checks can be configured per trial to issue automatic warnings or to show/hide questions when relevance depends on the context.
Online Queries
Issue and reply to queries from within the system for faster turnaround times. The query tool can be used by QA and/or trial monitors to streamline the process and increase efficiency.
In-form query resolution improves data accuracy and streamlines the QA/monitoring process.
One
Extensive optional configurations per clinical trial study.
Design your trials
Design CRFs from within the system
Add powerful edit Checks to the CRF
The Editor Module is aimed at CROs who want to have full control over the CRF implementation.
If you prefer, our trained staff can take care of this part for you and provide you with the eCRF to fit the study's needs.
CRF Design allows you to design the eCRF, from how many modules (eg. Visits) the trial will have to the questions that will appear on each page.
Edit Checks can be created for each question, triggering various types of responses: show warnings to the user, show/hide other questions and answer other questions automatically.
Compliance
21 CFR Part 11 Compliant
Exportable Audit trail
Several user access levels
By complying with regulatory requirements for electronic systems the EDC replaces paper based records.
A thorough audit trail system registers every change in the system.
A flexible authentication and authorization system allows restricting access to the system and to the functionalities each user can perform.
By collecting data as electronic source data, transcription errors decrease and trial data accuracy is optimized.
Tailoring to your needs
We provide various deployment options, depending on your infrastructure:
Technology Transfer run system on your network
Hosted solution use system in the cloud
ITClinical can adapt its base product to meet specific requirements, tailoring the application to your needs.
Instead of offering a fully fledged solution, we try to understand what you need and provide you with a customized, cost-effective solution.
Running on Linux servers, this also means there will be no increased licensing costs.
Phase I
eCRF library for re-use
Real-time sample collection times
Volunteer database
Phase I trials possess different challenges when EDC is concerned. With shorter life-cycles and similarities between studies, CROs can take advantage of our Phase I module to improve productivity in the fast-paced Phase I world.
eCRF libraries
With the eCRF library you can build your eCRF templates and use them multiple times as the base for new CRFs with a click of a button. This allows you to set up a new clinical trial in minutes instead of days!
Real-time data collection
Retrieving sample collection times is performed in fast-paced conditions - usually with seconds between each sample collection for a volunteer group.
The usual eCRF view per subject is not adequate to these conditions. With our Phase I module, you will be able to see subject data grouped by collection point, so you can use the EDC in real-time, on the ground as you're collecting the samples!
Volunteer database
An optional volunteer database can help you keep track of all your volunteer data. This means faster access to volunteers and the ability to reduce data management of information you've already collected.
