
Features
- Centralizes drug accountability workflow
- Eases the process of data retrieval
- Precise product traceability
- CFR 21 Part 11 Compliance
The IMPO (Investigational Medicinal Products Online) system is a drug accountability tool for the company's investigational medicinal products. It helps retrieving relevant product information and achieve drug accountability and enables real-time shipment record creation.
This tool is part of ITClinical's pharmaceutical portfolio and it incorporates the company's philosophy: making life easier for our partners.
By complying with regulatory requirements for electronic systems the EDC replaces paper based records.
- Collaborate online for faster feedback
- Access your data from anywhere
- Reduce overall clinical trial data management time
Highlights
Drug accountability
Product and batch management
Query all drug movements
Add extra files
Instant accountability and form generation
Associate with Clinical Trials
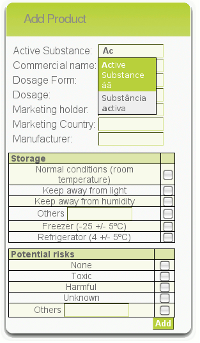
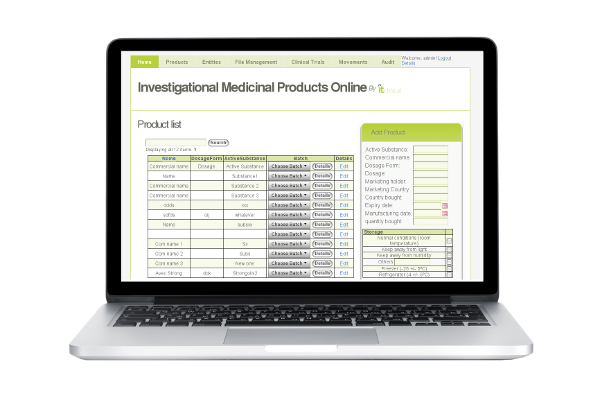
Products and batches can be added and managed within the IMPO system. The system manages basic information, such as product storage conditions and manufacturer.
Once the product is registered, users can record product/device shipments, thus allowing unitary traceability of dosage forms or devices. Queries can be performed to quickly retrieve information from the system.
Attach files
Extra files can be added to a drug accountability page - useful to quickly access information such as External package or patient leaflet information.
Product Shipment Forms
Simple product shipment forms can be generated on the fly so they can be added to the shipment package.
Clinical Trials
The IMPO system has a simple Clinical Trial management module. By registering very basic Trial information, medication/devices can be associated and queried by clinical trial.
Compliance
21 CFR Part 11 Compliant
Exportable Audit trail
Several user access levels
By complying with regulatory requirements for electronic systems it replaces paper based records.
A thorough audit trail system registers every change in the system.
A flexible authentication and authorization system allows restricting access to the system and to the functionalities each user can perform.
Tailoring to your needs
We provide various deployment options, depending on your infrastructure:
Technology Transfer run system on your network
Hosted solution use system in the cloud
ITClinical can adapt its base product to meet specific requirements, tailoring the application to your needs.
Instead of offering a fully fledged solution, we try to understand what you need and provide you with a customized, cost-effective solution.
Running on Linux servers, this also means there will be no increased licensing costs.
